Contact
ONSENCOM INC.2-5-2, Motoyokoyama-cho, Hachioji-shi, Tokyo-to, 192-0063, JAPAN


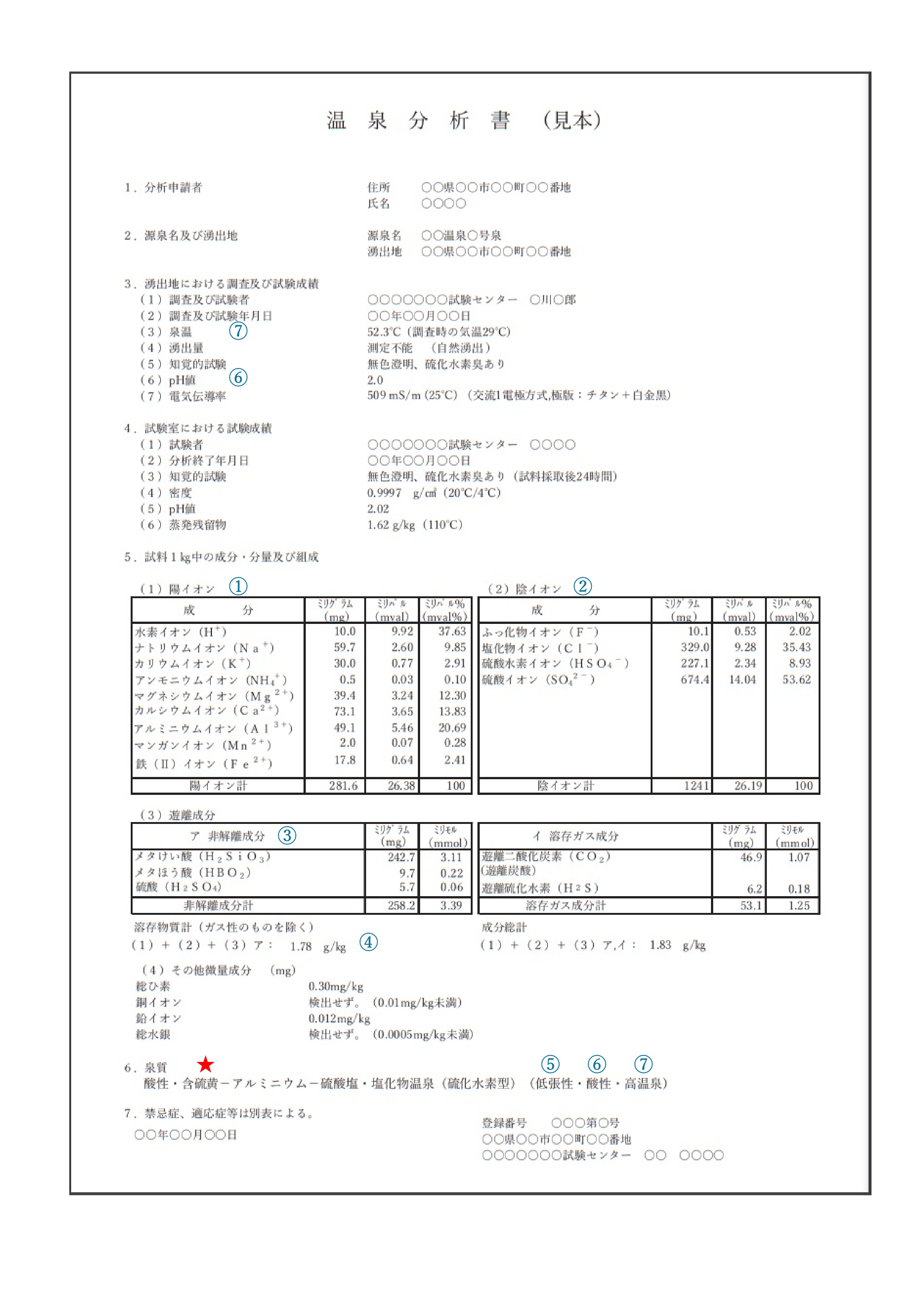
First, when looking for characteristics of “hot spring” from the “Hot Spring Analysis Reports”, the following 7 points are to be looked at.
① Cations + ② Anions + ③ Non-dissociated components = ④ Total Dissolved Substances (excluding gaseous substances)
⑤ Osmotic pressure ⑥ Hydrogen ion concentration (pH) ⑦ Spring temperature
The “spring quality name” is basically described as follows.
A:Special components―B:Cations―C:Anions D:Spring temperature
(F:Osmotic pressure G:Hydrogen ion concentration/Liquidity H:Classification by temperature)
↓
【★Example of spring quality name below】(pH8.6 with spring temperature of 33°C)
Acidic, Sulfur containing-Aluminum-Sulfate/Chloride springs
(Hypotonic, Acidic, High temperature spring)
The notation rules for spring quality name are described in detail in “1. Definition and Classification of Mineral Springs (pages 1-8)” at the beginning of the “Guidelines to the Mineral Spring Analysis Methods”.
Examples of “Hot Spring Analysis Reports” are described “8-6 Cautions for writing Hot Spring Analysis Reports” (pages 155-158).
鉱泉分析法指針
Guidelines for the Mineral Spring Analysis Methods
https://www.env.go.jp/council/12nature/y123-14/mat04.pdf
The “Guidelines for the Mineral Spring Analysis Methods” lists 10 types of spring qualities as “Therapeutic hot springs”. Even if one of these qualities applies to the spring, it will be given the name of the spring.Only “Therapeutic hot springs” are allowed to have a spring quality name.
The rules for naming the 10 types of “Therapeutic hot springs” are as follows.
■If the temperature of the spring is 25℃ or higher, there are no other conditions for naming the spring, and the total dissolved matter (excluding gaseous substances) are less than 1,000 mg/kg, the spring is classified as “Simple hot springs”. If the spring is alkaline or weak alkaline, it is named “Alkaline simple hot springs” or “Weak alkaline simple hot springs”.
■For those 1,000 mg/kg or more, the main component of anions is described in the spring quality name.
■Cations and anions are listed in this order and connected by “ー” or “–” (dash).
【Example】If the main component of the cation is “sodium ion” and the main component of the anion is “chloride
ion”
→ “sodium―chloride springs”
【Example】If the main component of the cation is “magnesium ion” and the main component of the anion is
“hydrogen carbonate ion”
→ “magnesium―hydrogen carbonate springs”
【Example】If the main component of the cation is “calcium ion” and the main component of the anion is “sulfate
ion”
→ “calcium―sulfate springs”
■If the 'mval%' (milli valent percentage) of both cations and anions are 20% or more, the substances are listed in descending order of content. If there are multiple special components, cations, and anions, they are separated by “.” (middle black, middle dot) (in the case of Japanese notation). When written in English, special components and cations are separated by “,” (comma) and anions by “/” or “/” (slash).
【Example】sodium, calcium, magnesium―hydrogen carbonate/chloride springs
■The components reflected in the spring quality name as cations are as follows.
sodium ions → sodium magnesium ions → magnesium
calcium ions → calcium aluminum ions → aluminum
iron(Ⅱ,Ⅲ) ions → iron(Ⅱ,Ⅲ)
hydrogen → hydrogen ※When the name of spring quality is “Acidic springs” and there is no other component with 20 mval% or more, it is described.
■If the special components are the specified value or more, they are described as follows.
If the “free carbon dioxide” content is 1,000 mg/kg or more → “carbon dioxide springs”
If the total iron ion content is 20 mg/kg or more → “iron containing springs”
If the hydrogen ion content is 1 mg/kg or more → “acidic springs”
If the iodide ion content is 10 mg/kg or more → “iodine containing springs”
If the total sulfur content is 2 mg/kg or more → “sulfur springs”
If the radon content is 8.25 mache/kg or more → “weak radioactive springs” / 50 mache/kg or more → “radioactive springs”
■If the special components (free carbon dioxide, total iron ions, hydrogen ions, iodide ions, total sulfur, and radon) are at the specified value or more, they are described at the beginning of the spring quality name.
【Example】sulfur containing ― sodium―chloride springs
If two or more special components are contained, they are described as follows. And the order of the special components is, in principle, as follows.
1. hydrogen ion (cation) → acidic
2. total sulfur[HSー+ S2O32ー+ H2S] → sulfur containing
3. free carbon dioxide → carbon dioxide containing
4. radon → radioactive containing
5. total iron ions [Fe²⁺+Fe³⁺] → iron containing
6. iodide ions → iodine containing
■【Example】acidic, sulfur containing ― sodium ― sulfate springs
weak radioactive containing, iron (Ⅱ,Ⅲ) ― magnesium ― sulfate springs
carbon dioxide containing, iron (Ⅱ,Ⅲ) ― simple cold mineral springs
sulfur containing, weak radioactive ― alkaline simple hot springs
■If it is not “Saline springs”, and the dissolved components are less than 1,000 mg/kg while the special components are equal to or more than the specified value, it is described as follows.
【Example】simple carbon dioxide springs
【Example】simple iron containing springs
【Example】simple acidic springs
【Example】simple iodine containing springs
【Example】simple sulfur springs
【Example】simple radioactive springs / simple weak radioactive springs
Other notation rules are as follows.
■Substances classified under the “Hot Springs Act” are contained at or above the specified value, and if the spring temperature is 25℃ or higher, it is called a “hot spring”;
if the temperature is less than 25℃, it is called a “cold mineral spring”.
【Example】If the temperature is 25℃ or higher・・・“sodium chloride spring” or “sodium chloride hot spring”.
※Other examples include “hydrogen carbonate springs” or “hydrogen carbonate hot springs” and “sulfate springs” or “sulfate hot springs” but most of them do not use the “hot” word.
If the temperature of the spring needs to be emphasized, “hot” word to be added.
【Example】If the temperature is less than 25℃・・・“calcium sulfate cold mineral springs”
■In the “classification by osmotic pressure, hydrogen ion concentration/pH, and spring temperature,” which is written alongside the spring name, the spring temperature should be classified as follows.
Less than 25℃・・・cold mineral spring
25℃ or higher to less than 34℃・・・low temperature spring
34℃ or higher to less than 42℃・・・hot spring
42℃ or higher・・・high temperature spring
■If it is a hot spring under the “Hot Springs Act” but is not classified as a therapeutic hot spring (when the metasilicic acid content is more than the specified value for a hot spring), it should be described as follows.↓
→It qualifies as a hot spring according to the metasilicic acid (H2SiO3) section in the appendix to Article 2 of the “Hot Springs Act”. However, it does not qualify as a therapeutic hot spring, and there is no name of the spring quality.
→It qualifies as a “hot spring (containing metasilicic acid)” under Article 2 of the “Hot Springs Act”.
Example of notation①: Spring temperature 52℃, pH 2.3, total dissolved substances 1000 mg/kg or more
A:Special components B:Cations C:Anions D:Spring temperature E:Classification of sulfur spring
F:Osmotic pressure G:Hydrogen ion concentration/liquidity H:Classification by spring temperature
Acidic, Sulfur-containing, Iron(Ⅱ,Ⅲ)―Calcium―Sulfate hot springs (Hydrogen sulfide type)
A B C D E
(Hypotonic, Acidic, High temperature spring)
F G H
※D: If the temperature is less than 25℃, it is called a “cold mineral spring”. The name “sulfate springs” can also be used here.
※E: “Sulfur spring” can be classified into “Sulfur type” and “Hydrogen Sulfide type”, but only
“Hydrogen Sulfide type” is indicated at the end.
※F, G and H: classification should be written together with the spring name.
※H: “spring temperature” of 42℃ or higher is indicated as “High temperature spring”.
Example of notation②: Spring temperature 41.2℃, pH 6.4, total dissolved substances 1000 mg/kg or more
A:Special components B:Cations C:Anions D:Spring temperature E:Classification of sulfur spring
F:Osmotic pressure G:Hydrogen ion concentration/liquidity H:Classification by spring temperature
Calcium, Sodium―Sulfate/Hydrogen carbonate/Chloride hot springs
B C D
(Hypotonic, Neutral, Hot spring)
F G H
※D: “hot springs” can be changed to “springs”.
※B, C: If there is more than one substance with 20 mval% or more of cations and anions, they are listed in descending order of abundance.
※H: If the temperature is 34℃ or higher to 42℃, it should be described as “Hot spring”.
Example of notation③: Spring temperature 25.4℃, pH 8.4, total dissolved substances less than 1,000 mg/kg
A:Special components B:Cations C:Anions D:Spring temperature E:Classification of sulfur spring
F:Osmotic pressure G:Hydrogen ion concentration/liquidity H:Classification by spring temperature
Weak Alkaline Simple hot springs
(Hypotonic, Weak alkaline, Low temperature spring)
F G H
※If the temperature of the spring is 25℃ or higher and the total dissolved substances (excluding gaseous substances) is less than 1,000 mg/kg, the spring is classified as “Simple hot springs”.
※If the spring is alkaline, the name of the spring is described as “Alkaline simple hot springs”.
※H: If the “spring temperature” is 25℃ or higher to less than 34℃, the spring is described as a “Low temperature spring”.
Example of notation④: Spring temperature 15.1℃, pH 8.6, total dissolved substances 1000 mg/kg or more
A:Special components B:Cations C:Anions D:Spring temperature E:Classification of sulfur spring
F:Osmotic pressure G:Hydrogen ion concentration/liquidity H:Classification by spring temperature
Sodium―Chloride cold mineral springs
B C D
(Hypotonic, Alkaline, Cold mineral spring)
F G H
※D: If the temperature of the spring is less than 25℃, it is described as a “cold mineral spring”.
※H: If the “spring temperature” is less than 25℃, it is described as a “cold mineral spring” instead of a “low temperature spring”.